1.Non toxic,Non inflammable
SF6 gas is currently the most excellent insulation and arc extinguishing medium, with much better insulation and arc extinguishing performance than insulation oil.
Its molecular structure consists of six fluorine atoms at the vertex positions, with a sulfur atom at the center, forming a fully symmetrical covalent bond structure known as a regular octahedron.
According to EEA (European Environment Agency).At environmental temperatures, sulfur hexafluoride is a colorless, odorless, non-toxic gas of high chemical stability and inertness. It is also non-flammable and about 5 times heavier than air, one of the heaviest known gases.
2.Man Made
In the 20th century, sulfur hexafluoride (SF6) was obtained by Moissan and Lebeau through the combustion of sulfur in fluorine gas. In the 1930s, Schub and Gamble proposed methods for producing SF6. Around the same time, the United Kingdom suggested using SF6 in transformers. In 1942, the Soviet Union also used SF6 in cables and batteries. Industrial production of SF6 began in the United States. Countries such as the United States, the United Kingdom, France, Germany, Italy, Russia, and Japan can produce SF6. In particular, the Japanese companies Kanto Denka and Asahi Salt, subsidiaries of the Electrochemical Corporation, had a production capacity of 1,000 tons per year in the 1970s.
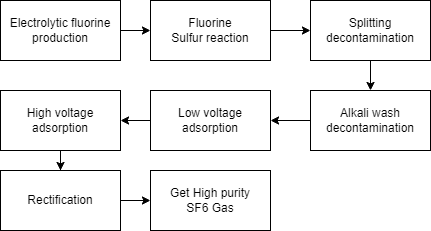
The process for making SF6 involves the reaction of sulfur and fluorine. Here is a general outline of the process:
Sulfur is heated to a high temperature, typically around 500-600 degrees Celsius.
Fluorine gas is introduced to the heated sulfur, and the two react to form SF6.
3.Life time- Very long (3200 years)
SF6 gas is highly stable. Its molecular structure consists of six fluorine atoms at the vertex positions, with a sulfur atom at the center, forming a fully symmetrical covalent bond structure known as a regular octahedron. It generally does not undergo chemical reactions at normal temperatures and even within the maximum allowable temperature range of electrical equipment (up to 150°C). Above 150°C, it may exhibit weak chemical reactions with certain plastics, while at temperatures above 200°C, it slowly reacts with copper and aluminum. SF6 gas starts to decompose at temperatures around 500°C.
According to US EPA info SF6 is a very stable chemical, with an atmospheric lifetime of 3,200 years. As the gas is emitted, it accumulates in the atmosphere in an essentially un-degraded state for many centuries. Thus, a relatively small amount of SF6 can have a significant impact on global climate change.
4.Better dielectric strength.Insulating properties 2.5 times that of air.
SF6 is a highly electronegative gas, and its molecules readily adsorb free electrons, forming large mass negative ions. This weakens the collision ionization process in the gas, resulting in a high electrical insulation strength. In a uniform electric field, the insulation strength of SF6 is approximately 2.5 times higher than air’s. SF6 gas exhibits a peak of thermal decomposition around t≈2000K. Therefore, during the zero-crossing of the alternating current arc, SF6 provides a much stronger cooling effect on the arc path than air. Its arc extinguishing capability is approximately 100 times that of air.
5.Five times heavier than air.
The density of sulfur hexafluoride is approximately five times that of air. Therefore, when a person inhales sulfur hexafluoride and speaks, the wavelength of the mechanical waves will become longer, making their voice sound more like that of a male.
6.Greenhouse gases
With the increasing attention to global climate change, terms such as greenhouse gases, energy efficiency, emission reduction, and low-carbon living are appearing more frequently in the public domain. In the public’s general understanding, carbon dioxide is considered the main greenhouse gas and the primary cause of global warming. However, from a scientific perspective, sulfur hexafluoride is the most potent greenhouse gas known to humans, with a warming potential 23,900 times higher than carbon dioxide. Controlling the uncontrolled release of sulfur hexafluoride gas is of utmost urgency. Therefore, in addition to intensifying research and development efforts on new insulation materials, it is also necessary to enhance public awareness of sulfur hexafluoride and strengthen regulatory measures in major industries and sectors where it is used to collectively address the challenges posed by sulfur hexafluoride in global climate change.
The “Kyoto Protocol,” adopted during the Third Session of the Conference of the Parties to the United Nations Framework Convention on Climate Change held in Kyoto, Japan, in 1997, explicitly stipulates the reduction of six greenhouse gases, including the following mentioned: [1] carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6). Among them, the latter three gases have the highest potential for causing the greenhouse effect. However, in terms of their contribution to global warming as a percentage.
About 80% of the global SF6 is used as an insulating medium in gas-insulated switchgear (GIS). The global annual emissions of SF6 are approximately 8,100 tons, equivalent to the carbon dioxide emissions produced by about 100 million new cars each year. Furthermore, SF6 has an atmospheric lifetime of at least 1,000 years.
International attention has been given to the emissions of SF6, and various countries have introduced policies to regulate its use and strengthen the recovery and reuse of SF6 gas.

The above are 6 thing you should know about SF6 Gas Properties. Base the properties.ensuring SF6 gas safe and effective use is what we are currently working on.






